Background
This is part of a release of experimental data determined at AstraZeneca on a set of compounds in the following assays: pKa, lipophilicity (LogD7.4), aqueous solubility, plasma protein binding (human, rat, dog , mouse and guinea pig), intrinsic clearance (human liver microsomes, human and rat hepatocytes).
Assay Information
Hepatic metabolic stability is a key pharmacokinetic parameter in drug discovery. Metabolic stability is usually assessed in microsomal fractions and only the best compounds progress in the drug discovery process.
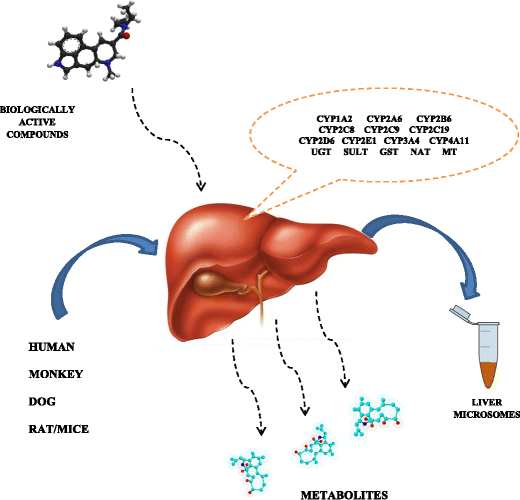
Image is from this paper. Biologically active compounds can be transformed or destroyed by the action of enzymes in the liver. Microsomes are small membrane bubbles (vesicles) that come from a fragmented cell membrane, and can be used as a proxy for how well a drug survives a trip through the liver.
Description of readout:
- HLM_CLEARANCE: Intrinsic clearance measured in human liver microsomes following incubation at 37C. Experimental range <3 to >150 microL/min/mg. Rapid Commun. Mass Spectrom. 2010, 24, 1730-1736.
Data resource
Reference: https://www.ebi.ac.uk/chembl/document_report_card/CHEMBL3301361/
Raw data: https://www.ebi.ac.uk/chembl/assay_report_card/CHEMBL3301370/